Do you mean they hadn't contacted you to start the verification process?Has anyone had Auricle get back to them regarding investing? We did not receive a response. We would meet the criteria to invest.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
New University of Michigan Tinnitus Discovery — Signal Timing
- Thread starter Hudson
- Start date
More options
Who Replied?- Dec 7, 2018
- 573
- Tinnitus Since
- 03/2018
- Cause of Tinnitus
- Unknown
Hah! I wish I did know that initial donor, I'll work on that... And that's awesome. I love hearing it. There's a famous movie line here in the states..."If you build it, he will come." I believe that the existence of an endowment could help secure its funding, and it also provides a convenient "consumer choice" option (I wouldn't worry too much about cannibalization between the two).Yes, it's something we've talked about. An endowment would be a great way to create steady, long-term funding for research. The challenge is that it only really makes sense once there's a big enough starting amount. For example, a $1 million fund earning around 4 to 5 percent per year would generate about $40,000 to $50,000 annually for research. With smaller amounts, the yearly return just isn't enough to make a real impact. For now, we're focused on growing our donor base, but once we reach that level, it's definitely something we'd like to set up.
Help us find that big initial donor
Tinnitus Quest, in my opinion, is what everyone has been waiting for. It has demonstrated accountability, transparency, and a clear vision and mechanism for advancing tinnitus research. You never know when someone out there catches wind of it and wants to leave a lasting legacy that will endure and see this mission through. The future is hopeful.
DimLeb
Member
- Jun 20, 2021
- 411
- Tinnitus Since
- 03/2021
- Cause of Tinnitus
- Idiopathic Cochleopathy or Maybe Loud Music
How is that? Doesn't every company out there, even medical ones like the Hough Ear Institute, ask for donations? Even the University of Michigan itself keeps sending me emails asking for donations after I gave just 10 euros on the page they had for Susan Shore.Auricle can't accept money from just anyone. The FDA could view that as pre-marketing, which is not something a company wants to risk.
If you're an accredited investor, you can invest in them. The minimum investment is $50,000. Go for it now.
The minimum investment is $50k, I think. It's not something the average tinnitus patient could invest in, and there's no guarantee it will show positive results in another trial.Has anyone had Auricle get back to them regarding investing
- Jan 16, 2020
- 616
- Tinnitus Since
- 1992
- Cause of Tinnitus
- noise? infection? negative stress? other?
Duh… I thought the Bionic Lab Institute of Australia, in Adelaide, I think, had already taken care of that. There's a lengthy thread about it here. Is there some kind of "Chinese wall" between the various researchers or what?It becomes clear that we need an objective tinnitus test to measure its existence and loudness. As long as we do not have that, it will remain too difficult, if not impossible, for anyone to develop a treatment that can reliably pass clinical trials.
I had hoped the Shore device would work and become available soon, but now I am not even sure about the first one.
FYI:
The Bionics Institute Claim They Have Found a Way of Objectively Measuring Tinnitus
Dr Mehrnaz Shoushtarian claims she has found a way to objectively measure tinnitus severity and whether a person has tinnitus or not via fNIRS (functional near-infrared spectroscopy). I’ve read a few articles on this already. I’ll link two below: Technology lets clinicians objectively detect...
- Oct 24, 2017
- 871
- Tinnitus Since
- 10/2017
- Cause of Tinnitus
- one-sided hearing loss (of unknown origin)
Thank you for your kind words, love it!Hah! I wish I did know that initial donor, I'll work on that... And that's awesome. I love hearing it. There's a famous movie line here in the states..."If you build it, he will come." I believe that the existence of an endowment could help secure its funding, and it also provides a convenient "consumer choice" option (I wouldn't worry too much about cannibalization between the two).
Tinnitus Quest, in my opinion, is what everyone has been waiting for. It has demonstrated accountability, transparency, and a clear vision and mechanism for advancing tinnitus research. You never know when someone out there catches wind of it and wants to leave a lasting legacy that will endure and see this mission through. The future is hopeful.
I am doubtful, though, on the "build it and they will come" quote. Maybe I misunderstand what you're saying, but the HUGE challenge we face is reaching a) as many people as possible, which takes major marketing efforts, and b) reaching the right people who can significantly propel the cause forward. Simply offering an endowment option will not make a difference unless we continue to make gargantuan efforts to reach a wide audience.
We recently reached the milestone of 10k followers on Instagram, which may not sound like much, but it's more than any of the other tinnitus and hearing charities. That took 1000s of hours of work to accomplish (no exaggeration).
Sorry, I don't mean to berate you at all, I just want people to understand how big the mountain is that we're trying to climb. It's the same reaction I always have when people (for the millionth time) tell us, why don't you "just" get a celebrity on board.

So please everyone, do think about who you might know in your network that could help connect us to some major donors! (Probably should be posting this in the Tinnitus Quest thread, haha.)
For the record, though, I too am hopeful that we will pull this off!
cjbhab
Member
A good reason why they should probably respond to those who do inquire. It is definitely a high-risk investment.The minimum investment is $50k, I think. It's not something the average tinnitus patient could invest in, and there's no guarantee it will show positive results in another trial.
With all due respect, and just to clarify, the FDA doesn't control private fundraising or investment. They only regulate the marketing and sale of unapproved devices. So, a company can accept funding from investors before approval; it's only public promotion or selling that's restricted. It's easy to see why people confuse the two.Auricle can't accept money from just anyone. The FDA could view that as pre-marketing, which is not something a company wants to risk.
If you're an accredited investor, you can invest in them. The minimum investment is $50,000. Go for it now.
I would gladly invest or donate a large sum, far more than $50k, if I believed it would help or speed up progress. My question is whether they actually need the money, or if there are other underlying reasons why it's taking so long to reach the market. Why haven't there been any recent trials? Are they concerned it won't perform well again?
Also, the device itself is relatively simple in both concept and components, which could make it vulnerable to imitation once released. Perhaps part of the delay has to do with ensuring proper copyright protection and a solid market strategy before rollout. Still, I hope the main focus remains on bringing relief to those who are suffering, rather than getting caught up in red tape or commercial interests.
Just to clarify the Phase 2 trial results: the bisensory treatment (auditory and somatosensory) produced significant improvements in TFI scores and tinnitus loudness. The sound-only phase didn't show any meaningful benefit, so the positive effects are specifically linked to the bisensory approach.Further trials were mentioned in the presentation and again at the end during the investors' pitch. The point is that the data from Phase 2 did not show a clear benefit. The second group did not experience improvement from the treatment compared to the sound-only (placebo) phase.
Of all the posts I've seen, only @UKBloke hasn't buried his head in the sand. If I presented the same Phase 2 results, but for a different condition such as depression, would you look at them and call them positive, and therefore wave them through for approval?
It's definitely good to evaluate the results critically, but the published data support that bisensory stimulation was effective compared to sound-only treatment. So, in answer to your question, yes, I would approve it based on the trial data.
- Oct 17, 2017
- 663
- Tinnitus Since
- 2005
- Cause of Tinnitus
- unknown - possibly hereditary
With all due respect, you're mostly wrong.With all due respect, and just to clarify, the FDA doesn't control private fundraising or investment. They only regulate the marketing and sale of unapproved devices. So, a company can accept funding from investors before approval; it's only public promotion or selling that's restricted. It's easy to see why people confuse the two.
I would gladly invest or donate a large sum, far more than $50k, if I believed it would help or speed up progress. My question is whether they actually need the money, or if there are other underlying reasons why it's taking so long to reach the market. Why haven't there been any recent trials? Are they concerned it won't perform well again?
You're right, the FDA doesn't regulate who a company can raise money from, only how the device is marketed. So, private fundraising from accredited investors is fine, but public crowdfunding that promotes or pre-sells an unapproved device would likely violate FDA rules on pre-approval marketing. Auricle does not want to take that risk. This is why they only take money from accredited investors.
If you have access to such large wads of cash (I assume you would then meet the accredited investor requirement), why not reach out to them and make an investment offer? They would probably share more information with a potential investor than a random forum user. To me, it sounds like you're just one person in a long line of people who like to run their mouth.
While you're at it, give some of your cash to Tinnitus Quest. I dare you!!!
Appreciate the enthusiasm, but just to clarify — I never mentioned crowdfunding or pre-selling. I was referring to legitimate investment or partnership options, which have nothing to do with FDA marketing rules.With all due respect, you're mostly wrong.
You're right, the FDA doesn't regulate who a company can raise money from, only how the device is marketed. So, private fundraising from accredited investors is fine, but public crowdfunding that promotes or pre-sells an unapproved device would likely violate FDA rules on pre-approval marketing. Auricle does not want to take that risk. This is why they only take money from accredited investors.
If you have access to such large wads of cash (I assume you would then meet the accredited investor requirement), why not reach out to them and make an investment offer? They would probably share more information with a potential investor than a random forum user. To me, it sounds like you're just one person in a long line of people who like to run their mouth.
While you're at it, give some of your cash to Tinnitus Quest. I dare you!!!
As for "running my mouth off," I'd call it a discussion — that's what forums are for. Investing on a dare, though? That's bad business; I'll pass on that strategy.
My main point is trying to understand the delay with the Susan Shore device and whether funding or resources could help get it to those who need it faster. It seems likely there are factors beyond money — regulatory strategy or internal priorities — that haven't been publicly disclosed.
@Hotrock, the group that had sound first, followed by a washout, did not improve with treatment.The sound-only phase didn't show any meaningful benefit, so the positive effects are specifically linked to the bisensory approach.
another_person
Member
- Oct 22, 2025
- 19
- Tinnitus Since
- december 2024
- Cause of Tinnitus
- T1: Acoustic Trauma. T2: Medication/Supplements interaction
- Jul 8, 2019
- 1,214
- Tinnitus Since
- 1991
- Cause of Tinnitus
- Loud Music / family history
This is definitely a discussion thread, but to be fair, you can only understand the delay if you acknowledge the problem within your own statement:My main point is trying to understand the delay with the Susan Shore device and whether funding or resources could help get it to those who need it faster.
That statement isn't conclusive because their own published data also indicates that the device isn't nearly as effective if preceded by a control.the published data support that bisensory stimulation was effective compared to sound-only treatment.
I accept that within the bounds of the scientific process, they have a legitimate reason to dismiss the problematic data; however, there are two loudness data sets in each arm, and by only focusing on the one that spins the cross-over effect positively, their current position became untenable in my view. I'm not suggesting they can't move forward, but to date, they haven't been entirely clear about how they will do that.
Anyhow, I think acknowledging the whole of the loudness data set frames the funding question in a more realistic context.
Interesting interpretation, but I think this might be simpler than it seems. The team didn't "spin" the results; they left out the second-period data because of a carryover effect, which is completely standard in crossover studies. If anything, that carryover suggests the treatment continued working beyond the washout, which actually strengthens the case. My hunch is that the delay has much more to do with regulatory and commercial timing than anything hidden in the loudness tables.This is definitely a discussion thread, but to be fair, you can only understand the delay if you acknowledge the problem within your own statement:
That statement isn't conclusive because their own published data also indicates that the device isn't nearly as effective if preceded by a control.
I accept that within the bounds of the scientific process, they have a legitimate reason to dismiss the problematic data; however, there are two loudness data sets in each arm, and by only focusing on the one that spins the cross-over effect positively, their current position became untenable in my view. I'm not suggesting they can't move forward, but to date, they haven't been entirely clear about how they will do that.
Anyhow, I think acknowledging the whole of the loudness data set frames the funding question in a more realistic context.
One group received the bimodal treatment first, while the other group received sound-only treatment first, with a four-week washout period in between.@Hotrock, the group that had sound first, followed by a washout, did not improve with treatment.
The "sound-first" group did later receive the bimodal treatment and showed improvement, but because of a strong carryover effect, the authors only analyzed the first treatment period in detail.
So it's not that they "didn't improve"; it's that the data couldn't be used cleanly for statistical comparison. It's an easy mistake to make if you skimmed the paper, but the nuance matters.
You would need to check, but from what I understand, the treatment works best when it is matched to your tinnitus frequency. It may still provide some benefit even if you cannot match it perfectly, though I wouldn't take my word for it without confirming.I read the Tinnitus Hub interview with Susan Shore and realized that despite my crippling tinnitus, I'll never be a candidate for her treatment since I can't hear my tinnitus frequency due to high-frequency hearing loss. I guess I won't be wasting money on Lenire either.
- Jul 8, 2019
- 1,214
- Tinnitus Since
- 1991
- Cause of Tinnitus
- Loud Music / family history
There's nothing hidden in that loudness chart. On the contrary, it's very clear for those willing to see it; the control didn't wash out either and exerted a negative effect on the treatment. Repeating their own spin won't change that fact.they left out the second-period data because of a carryover effect, which is completely standard in crossover studies. If anything, that carryover suggests the treatment continued working beyond the washout, which actually strengthens the case. My hunch is that the delay has much more to do with regulatory and commercial timing than anything hidden in the loudness tables.
Hey, I see what you're trying to argue, but if you take a proper look at the Phase 2 data from Susan Shore's trial, the picture isn't nearly as mysterious as you're making it.There's nothing hidden in that loudness chart. On the contrary, it's very clear for those willing to see it; the control didn't wash out either and exerted a negative effect on the treatment. Repeating their own spin won't change that fact.
The bisensory treatment (auditory and somatosensory) showed a clinically meaningful TFI reduction of about 12 points at week 6 (p < .001). That's solid, both statistically and clinically. The sound-only arm barely reached a reduction of 4 points, which the authors themselves described as "little effect," definitely not clinically meaningful.
As for the "carryover," that's already explained in the paper. The bisensory effect lasted into the washout phase, which means the second crossover period couldn't be analyzed cleanly. That's not a flaw, it's a sign the treatment worked well enough to persist.
So when you say the control "didn't wash out" and somehow "negatively affected" the treatment, that's just not what the data shows. The results are clear: bisensory stimulation worked, sound-only didn't, and the only thing that carried over was the benefit.
In other words, this isn't "spin," it's peer-reviewed evidence. If we start rewriting the stats to fit a theory, we're not analyzing the data anymore.
That said, like you, I'm skeptical too, which is why I made my first post. But I also try to stay hopeful and positive. Right now, the Susan Shore approach remains one of the most promising options for all of us tinnitus sufferers.
- Jul 8, 2019
- 1,214
- Tinnitus Since
- 1991
- Cause of Tinnitus
- Loud Music / family history
There's no point in cutting and pasting TFI statistics because I'm only focusing on loudness reduction. Also, we don't need hunches, hope, or mystery. We need hard data.Hey, I see what you're trying to argue, but if you take a proper look at the Phase 2 data from Susan Shore's trial, the picture isn't nearly as mysterious as you're making it.
The bisensory treatment (auditory and somatosensory) showed a clinically meaningful TFI reduction of about 12 points at week 6 (p < .001). That's solid, both statistically and clinically. The sound-only arm barely reached a reduction of 4 points, which the authors themselves described as "little effect," definitely not clinically meaningful.
As for the "carryover," that's already explained in the paper. The bisensory effect lasted into the washout phase, which means the second crossover period couldn't be analyzed cleanly. That's not a flaw, it's a sign the treatment worked well enough to persist.
So when you say the control "didn't wash out" and somehow "negatively affected" the treatment, that's just not what the data shows. The results are clear: bisensory stimulation worked, sound-only didn't, and the only thing that carried over was the benefit.
In other words, this isn't "spin," it's peer-reviewed evidence. If we start rewriting the stats to fit a theory, we're not analyzing the data anymore.
That said, like you, I'm skeptical too, which is why I made my first post. But I also try to stay hopeful and positive. Right now, the Susan Shore approach remains one of the most promising options for all of us tinnitus sufferers.
One breakthrough in Susan Shore's work lay in the fact that it would finally (as much as possible by today's standards in science) provide a table of hard data on tinnitus volume reduction. It has to be remembered that this aspect of her research flew right in the face of Neuromod's (I'm assuming you're familiar with Neuromod's TENT-A2 & A3 papers?)
In 2018, Susan Shore et al published the protocol on their sham control (I also assume you're familiar with that paper?) They were unequivocal [my emphasis]:
None of the control stimuli (sedative alone, unimodal somatosensory or unimodal auditory stimulation) had any effect on tinnitus behaviors or tinnitus correlates.
By their own assertion, then, the active treatment in the second arm of the pivotal trial we're currently discussing should have behaved exactly, or at least very similarly to the active treatment in the first arm. It patently didn't, and one simply cannot randomly divorce an objective fact from reality to suit an argument. That is the very definition of spin.
For the record, for the final time, I'm not saying that the device does not work. What I am saying, though, is that we are in, by virtue of UMich's own loudness data, Schrödinger's uncertainty territory because as far as volume reduction is concerned, the device both works and it doesn't work. It's incumbent on UMich and Auricle to resolve that paradox.
You're clearly sticking to your guns, so I'm not going to labour this point any further with you; however, on the subject of comparing apples with oranges (TFI in a discussion about volume reduction), I'm curious to know: Neuromod has advertised the clinically significant TFI gains in their own research. You live in London, right? Lenire is available in Harley St, have you considered buying it?
withintention
Member
- Nov 26, 2023
- 133
- Tinnitus Since
- 10/2023
- Cause of Tinnitus
- Noise and/or infection
I actually have a theory about this.
If the device truly works, consider the lived experience of Group 1 (active treatment first) and Group 2 (sham treatment first) during this period.
If the device is effective, Group 1 experiences improvement while Group 2 does not during the first six weeks. Group 1 feels elated because their tinnitus has actually improved, while Group 2 feels disappointed because theirs has not. Again, this is assuming the device works.
In the next phase, after the washout period, Group 1 (now receiving the sham treatment) believes the treatment works. Group 2 (now receiving the active treatment) does not believe the treatment works.
This difference alone could have influenced how each group reported loudness during this phase.
I know that when I am at the audiologist for a hearing test, I tend to try harder to hear sounds—and even report more false positives—when I feel optimistic about the state of my tinnitus than when I feel pessimistic about it.
If the device truly works, consider the lived experience of Group 1 (active treatment first) and Group 2 (sham treatment first) during this period.
If the device is effective, Group 1 experiences improvement while Group 2 does not during the first six weeks. Group 1 feels elated because their tinnitus has actually improved, while Group 2 feels disappointed because theirs has not. Again, this is assuming the device works.
In the next phase, after the washout period, Group 1 (now receiving the sham treatment) believes the treatment works. Group 2 (now receiving the active treatment) does not believe the treatment works.
This difference alone could have influenced how each group reported loudness during this phase.
I know that when I am at the audiologist for a hearing test, I tend to try harder to hear sounds—and even report more false positives—when I feel optimistic about the state of my tinnitus than when I feel pessimistic about it.
Great, we should contact Auricle now that the matter can be put to rest.I actually have a theory about this.
If the device truly works, consider the lived experience of Group 1 (active treatment first) and Group 2 (sham treatment first) during this period.
If the device is effective, Group 1 experiences improvement while Group 2 does not during the first six weeks. Group 1 feels elated because their tinnitus has actually improved, while Group 2 feels disappointed because theirs has not. Again, this is assuming the device works.
In the next phase, after the washout period, Group 1 (now receiving the sham treatment) believes the treatment works. Group 2 (now receiving the active treatment) does not believe the treatment works.
This difference alone could have influenced how each group reported loudness during this phase.
I know that when I am at the audiologist for a hearing test, I tend to try harder to hear sounds—and even report more false positives—when I feel optimistic about the state of my tinnitus than when I feel pessimistic about it.
DimLeb
Member
- Jun 20, 2021
- 411
- Tinnitus Since
- 03/2021
- Cause of Tinnitus
- Idiopathic Cochleopathy or Maybe Loud Music
By November 2027, they will organise a Q&A, and by November 2029, they will actually answer, lol.Great, we should contact Auricle now that the matter can be put to rest.
In the right context, you could already say that they are almost market-ready.By November 2027, they will organise a Q&A, and by November 2029, they will actually answer, lol.
I completely agree. I think it is mainly that many people are suffering and have suffered for a long time. They just want the tinnitus gone and are frustrated with the timeframe. The reality is that progress happens slowly, but we should be grateful for the people who are working on it. It is not easy to do, especially in a strict regulatory environment, and if someone thinks it is simple, I suggest they solve the issue themselves and they will become millionaires in the process.I'm a bit shocked by the responses here. We've been given a timeline. In less than five years, the device will be on the market. I'm very grateful that they announced their timeline and kept it realistic. Hopefully things will go smoothly, and we'll be using it before the end of 2028.
I've been digging into this for a few weeks and have now exchanged several emails directly with Prof. Susan Shore. Below is the only conclusion that survives both the published graph and her personal replies.There's no point in cutting and pasting TFI statistics because I'm only focusing on loudness reduction. Also, we don't need hunches, hope, or mystery. We need hard data.
One breakthrough in Susan Shore's work lay in the fact that it would finally (as much as possible by today's standards in science) provide a table of hard data on tinnitus volume reduction. It has to be remembered that this aspect of her research flew right in the face of Neuromod's (I'm assuming you're familiar with Neuromod's TENT-A2 & A3 papers?)
In 2018, Susan Shore et al published the protocol on their sham control (I also assume you're familiar with that paper?) They were unequivocal [my emphasis]:
None of the control stimuli (sedative alone, unimodal somatosensory or unimodal auditory stimulation) had any effect on tinnitus behaviors or tinnitus correlates.
By their own assertion, then, the active treatment in the second arm of the pivotal trial we're currently discussing should have behaved exactly, or at least very similarly to the active treatment in the first arm. It patently didn't, and one simply cannot randomly divorce an objective fact from reality to suit an argument. That is the very definition of spin.
For the record, for the final time, I'm not saying that the device does not work. What I am saying, though, is that we are in, by virtue of UMich's own loudness data, Schrödinger's uncertainty territory because as far as volume reduction is concerned, the device both works and it doesn't work. It's incumbent on UMich and Auricle to resolve that paradox.
You're clearly sticking to your guns, so I'm not going to labour this point any further with you; however, on the subject of comparing apples with oranges (TFI in a discussion about volume reduction), I'm curious to know: Neuromod has advertised the clinically significant TFI gains in their own research. You live in London, right? Lenire is available in Harley St, have you considered buying it?
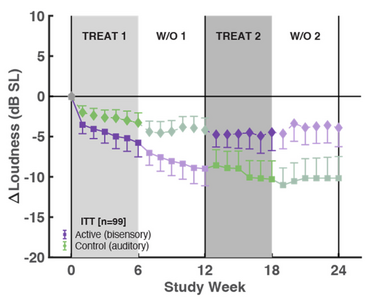
1. What the graph (eFigure 5) actually shows – approx measured, not exact:
Group 1 (active first)
End of active (Week 6): ≈ −9.5 dB
Week 24 (after sham + washout): ≈ −10 to −11 dB (they keep almost everything)
Group 2 (sham first → active second)
End of sham (Week 12): ≈ −2.8 dB
End of active (Week 18): ≈ −5 to −6 dB total from baseline
Week 24: still ≈ −5 dB total
Sham-first patients end up with only about 50-60% of the loudness reduction that active-first patients achieve, even though both groups received exactly 6 weeks of the real bimodal device.
2. Susan Shore's own words (direct quotes from her two emails to me when I questioned her last week):
17 Nov 2025
"Because the effect in period one was so effective, there was no recovery of loudness towards baseline during the washout period … Therefore, because the effects were still ongoing … we were unable to use the data from weeks 12-24. This is called a crossover effect. … Thus, for this study we only used period one."
18 Nov 2025
"There is also a crossover effect with the sham. Even tho the reduction in tinnitus loudness with the sham was much less than with the bimodal treatment – it also did not recover to baseline. Therefore … no data from the 12-24 week period can be used or interpreted."
She will not go further than that. She will never give the clear patient sentence "the bimodal treatment works just as well if you get it second after the sham", because that would require interpreting Period 2, which she correctly says is statistically invalid.
3. What this means in plain English:
The only statistically valid proof of efficacy is Period 1: ~9–10 dB reduction with active vs ~2.8 dB with sham.
Descriptively, patients who had prolonged sound-only (sham) exposure first ended up with a noticeably smaller final benefit than patients who got the real bimodal device first.
4. My personal takeaway (shared by quite a few of us now):
Prior exposure to any prolonged sound-only therapy (whether the exact sham stimulus used in the trial or years of Notch/Neuromod/Lenire/etc.) appears to blunt the subsequent bimodal effect.
If you want the best chance of the full ~10 dB+ reduction seen in the active-first arm, you probably need a decent wash-out period of no sound therapy at all before starting the Auricle device.
That is the most honest reading of the only randomised trial we have, backed by Susan Shore's own emails and the graph she published.
Feel free to quote or link this post. The data and the emails speak for themselves.
Attachments
- Jul 8, 2019
- 1,214
- Tinnitus Since
- 1991
- Cause of Tinnitus
- Loud Music / family history
Notwithstanding the fact that the sham induced a placebo effect, you can't statistically validate the existing Treatment 1 loudness chart without actually having designed the entire trial as a single-arm trial. Ergo, Susan Shore's statement about how well the active treatment performed in Treatment 1 cannot be validated either.The only statistically valid proof of efficacy is Period 1: ~9–10 dB reduction with active vs ~2.8 dB with sham.
Ken219
Member
Caught on to the sham?Notwithstanding the fact that the sham induced a placebo effect, you can't statistically validate the existing Treatment 1 loudness chart without actually having designed the entire trial as a single-arm trial. Ergo, Susan Shore's statement about how well the active treatment performed in Treatment 1 cannot be validated either.
Amen, brother.The only trial I care about is the trial on myself.

 Member
Member Director
Director